
Calibration & Compliance
Ensure long-term accuracy and maintain traceable calibration for your devices in regulated environments such as pharmaceutical, biotech, cold-chain, and food safety
Ensure long-term accuracy and maintain traceable calibration for your devices in regulated environments such as pharmaceutical, biotech, cold-chain, and food safety

Calibration & Compliance
Ensure long-term accuracy and maintain traceable calibration for your devices in regulated environments such as pharmaceutical, biotech, cold-chain, and food safety
Factory Calibration Certificate
Our devices include a factory calibration report valid for 2 years. After this period, customers who require traceable accuracy or need to meet compliance standards can choose from several calibration renewal options.

Traceability
Calibration performed using instruments traceable to national and international metrology standards

Compliance
Suitable for audits and regulatory frameworks (ISO, GMP, HACCP, GDP, FDA-related practices)
Note: Our devices use high-precision industrial-grade sensing elements. Over several years of continuous operation, small variations may occur due to the natural aging of electronic components, environmental stress, and long-term thermal cycles. This phenomenon is standard across all professional measurement instruments and does not indicate a malfunction.
Option 1: Renew Calibration at an Accredited Laboratory
You may send the device to any locally accredited calibration laboratory to obtain a new traceable calibration certificate.This is recommended for environments requiring strict compliance, including ISO, GMP, HACCP, or internal audits.

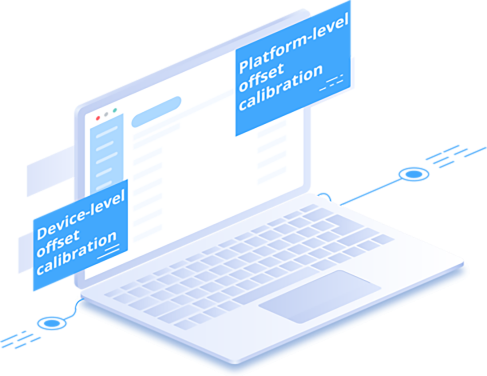
Option 2: Use the Platform's Built-In Self-Calibration Tools
Our platform includes built-in features for routine verification, allowing users with a certified reference instrument to apply offsets at both the platform and device level directly in the system without returning the device, suitable for internal QA/QC but not replacing an external calibration certificate.
Option 3: Start a New 2-Year Calibration Cycle
You may choose to purchase a new device, which includes a fresh 2-year factory calibration report.
While the device's overall service life remains long, precision components naturally experience gradual performance drift over extended periods of use. For applications requiring strict accuracy assurance or high audit readiness, some customers choose periodic device replacement as part of their risk-based calibration strategy.
Why Regular Calibration Matters
Maintaining proper calibration ensures stable accuracy, audit readiness, and long-term reliability—especially in regulated industries.

Standard environments:
every 18-24 months

Critical monitoring (pharmaceutical,
cold-chain): every 12-18 m

High humidity or harsh environments:
more frequent verification recommended
|
Products |
Dashboards |
Business Partners |
|
WS1 |
UbiBot Web Console |
Volume Pricing |
|
WS1 Pro |
UbiBot Space |
Become a Distributor |
|
GS1 |
UbiBot Support Desk |
Affiliates |
|
GS2 |
Agency Web Console |
|
|
MS1 |
||
|
SP1 |
||
|
Accessories |
|
Docs |
Purchase |
Company |
|
Platform API |
Pricing |
News |
|
Q&A |
UbiBot Partners |
About us |
|
Privacy Policy |
Online Store |
Contact |
|
Terms of Service |
System Status |